Mon, Jan 27, 2025
[Archive]
Volume 21, Issue 4 (December 2024)
IJMSE 2024, 21(4): 1-10 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Prasanna U, Kambila V K, Nadella K J. Effect of Fe2O3 Nano Filler on Structural and Electrical Properties of PVB-NaNO3 Complexed Solid Polymer Electrolytes for Electrochemical Cell Applications. IJMSE 2024; 21 (4) :1-10
URL: http://ijmse.iust.ac.ir/article-1-3650-en.html
URL: http://ijmse.iust.ac.ir/article-1-3650-en.html
Abstract: (4985 Views)
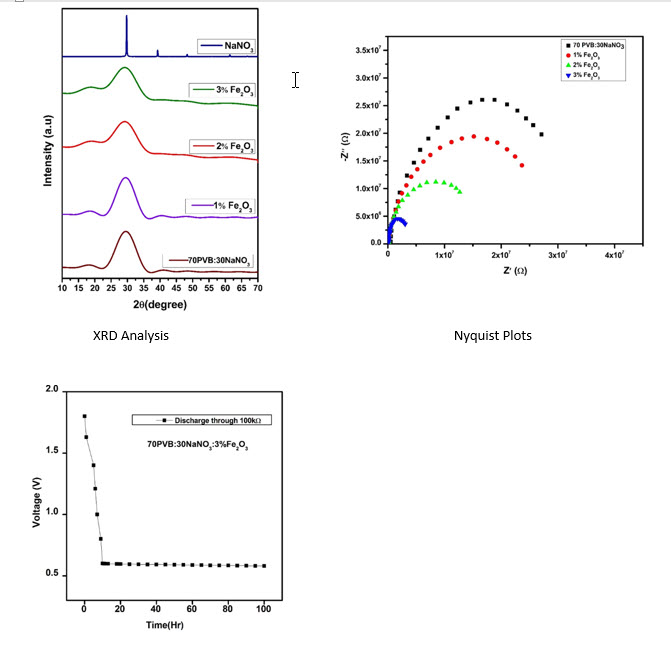
The composite solid polymer electrolyte films were prepared by doping nano-sized Fe2O3 particles on PVB (Polyvinyl Butyral) complexed with NaNO3 salt by solution casting technique. FTIR, XRD, and SEM methods characterized these electrolyte films. The Fourier Transform Infrared Spectroscopy and X-ray diffraction methods reveal the structural and complexation changes occurring in the electrolytes. The surface morphology of the electrolyte film was examined using the SEM (Scanning Electron Microscope) technique. The PVB+NaNO3+Fe2O3(70:30:3%) electrolyte shows a moderate ionic conductivity of 2.51×10−5 S cm−1 at ambient temperature (303 K). AC impedance spectroscopic analysis evaluates the ionic conductivity of the produced polymer electrolyte. Wagner's polarisation technique was applied to study the charge transport characteristics in the electrolyte films. The investigation revealed that ions constituted the majority of the transport carriers. An Open Circuit Voltage (OCV) of 2.0V and a Short Circuit Current (SCC) of 0.8 mA were found in the discharge characteristics data for the cell constructed with the polymer electrolyte sample.
Keywords: Polymer electrolytes, X-ray diffraction, Ionic conductivity, Discharge characteristics, Optical properties
Type of Study: Research Paper |
Subject:
Polymers
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |